Quality Index: (Clinical Approval No.: 2004L04244).(balofloxacin)
C
20H
24FN
3O
4.2H
2O
425.46
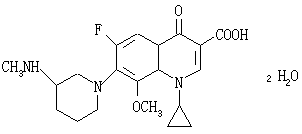
This product is 1-cyclopropyl-6-fluoro-8-methoxy-7-[3-(methylamino)-1-piperidinyl]-4-oxo-1,4-dihydro-3-quinoline carboxylic acid dihydrate, calculated as anhydrous, containing balofloxacin ( C20H24FN3O H24FN3O4 ) Not less than 99.0%.
[Property] White or light yellow crystalline powder
[Identification].
1. The chemical identification of this product is reddish-brown
2. The infrared spectrum of this product should be consistent with that of the reference substance
3. The HPLC diagram of this product should be consistent with the retention time of the main peak of the sample compared to the main peak of the control substance.
4. This product should show organic fluorine reaction
[Inspection]
The clarity of the solution is less than that of the No. 1 turbidity standard
The clarity and color are less than the No. 4 standard colorimetric solution
Moisture content should be 8.0%-8.9%.
Residue on ignition Not exceed 0.2%
Fluorine shall not be less than 4.40%.
Heavy metals must not exceed 20ppm
The impurities of the relevant substances are less than 1.0%.
[Content] calculated as anhydrous, containing Balofloxacin (C 20 H 24 FN 3 O 4 ) shall not be less than 99.0%.


