Lomefloxacin Hydrochloride
("New Drug Conversion Standards", Vol. 48, WS 1 -(X-513)-2003Z).
Product name: Lomefloxacin Hydrochloride
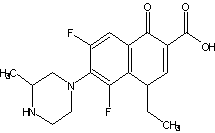
Chemical Structure:
Molecular Formula: C17H19F2N3O3
Molecular Weight: 387. 81
This product is (±)1-ethyl-6,8-difluoro-1,4-dihydro-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinoline carboxylic acid hydrochloride. Calculated as a dry product, the content of lomefloxacin (C17H19F2N3O3) shall not be less than 89.2%.
[Property] is white or off-white crystalline powder, almost odorless, bitter taste
[Identification].
(1) IR The infrared absorption pattern of this product should be consistent with the control pattern
(2) The retention time of the main peak of the HPLC test sample should be consistent with the retention time of the main peak of the control substance
(3) The aqueous solution of this product should show the identification of chloride
[Check].
The clarity and color of the solution should be in accordance with the regulations
The relevant substance shall not exceed 0.5% of a single impurity
The total impurities shall not exceed 1.0%.
Loss on drying Must not exceed 0.5%
Residue on ignition Must not exceed 0.1%
Heavy metals shall not exceed 20 parts per million
[Content determination] According to the dry product, the content of lomefloxacin (C17H19F2N3O3) shall not be less than 89.2%.